Tecan’s corporate values of trust, highest standards and ambition are the cornerstones of our business and provide the framework for Tecan’s culture. Our customers, investors and other stakeholders trust Tecan to act responsibly and ethically as we meet our commitments to them, and our strong corporate governance processes ensure that this trust is honored. As well as reaching highest standards with our products, we work to provide reliable high-quality service to our stakeholders across all business areas, ensuring their data is secure, business risks are anticipated and proactively managed, and that any feedback provided is responded to appropriately.
GOVERNANCE AND ETHICS
CODE OF CONDUCT
Tecan’s good governance and ethical practices are reflected in the Organizational Regulations and Tecan’s Code of Conduct, available on tecan.com. Tecan’s Code of Conduct is binding for all employees, managers and Board members. In this Code, Tecan undertakes to maintain the highest standards in its business activities and to respect ethical values. The Code of Conduct was drafted by Tecan's General Counsel in accordance with industry best practice standards. With the Code, Tecan aims to document internally and externally that the Company is a credible and reliable business partner and employer in all situations. The Code promotes compliance with standards on occupational health, safety and the environment, provides instructions on ensuring data protection and handling confidential information, and requires accurate and timely communication of information and careful logging of relevant meetings and processes by Tecan staff. The Code also stipulates compliance with competition law as well as national and international trade law for the import and export of products. It also includes a zero-tolerance policy toward bribery and corruption and guarantees anonymity for whistleblowers.
Line managers are responsible for ensuring that all their staff know and understand the content of the Code of Conduct. The Code is available in English and German as well as seven other languages, including Spanish, Chinese and Japanese. By providing these different language versions, Tecan wishes to ensure that this important document is understood by employees all around the world. All employees globally must attend and successfully complete a training course on the Code when joining Tecan, and then every two years following. As of 31 December 2024, 96.3% of all employees who have access to the Learning Services Organization (LSO) learning platform, and 75.4% of all contractors at Tecan who have access to the LSO learning platform, had completed the Code of Conduct training. Just over 40% of Tecan employees have access to a learning platform that is separate to the LSO and carry out their Code of Conduct training there. Our training % data currently doesn’t include these employees and we expect to have combined training data in future. For all employees, for more advice and guidance on the Code of Conduct employees are encouraged to discuss with their line manager, and concerns can be raised anonymously via the whistleblower hotline. Tecan’s LSO use is tracked per individual, and managers verify that assigned trainings have been completed regularly and at a minimum prior to each employee’s annual performance review discussion. Mapping of individual LSO training completion to each employee’s employee category and region is not carried out due to capacity constraints and a preference to address this topic at the individual level.
Tecan has established several organizational control mechanisms with the aim of ensuring good governance and ethical behavior. The Internal Audit department has the task of periodically assessing the effectiveness of the internal control system. The internal control system consists of all organizational measures taken by the Company in order to maintain the effectiveness of its operations, protect the corporate resources, appropriately manage the risks and ensure compliance with laws and regulations, while always keeping a strong focus on the trustworthiness of the financial reporting. Internal Audit has the power to check and verify processes, systems, management activities, projects and contracts, acting as a supervisory body independent from operations and is reporting directly to the Audit Committee of the Board of Directors. In the year under review, the Audit Committee and Internal Audit held several meetings. The department is subject to the international standards for internal auditing.
Tecan has not been involved in any significant instances of non-compliance with laws and regulations during the reporting period, including legal cases, rulings or other events related to corruption, bribery, anti-competitive behavior, anti-trust, or monopoly practices. No fines or non-monetary sanctions were imposed on Tecan or paid by Tecan in 2024, and no contracts with business partners were terminated due to violations related to corruption.
WHISTLEBLOWER HOTLINE
Tecan employees and third parties can report possible events of misconduct via a third party-managed whistleblower hotline, accessible at tecan.com. This whistleblower hotline also functions as a channel for filing grievances. This dedicated mailbox and multi-language telephone hotline is run by EQS, a specialized provider of compliance solutions. Reports can be filed anonymously if preferred and all complaints are reviewed by Tecan’s Compliance department, discussed with top management and addressed as necessary. The EQS platform ensures the highest standards of confidentiality and anonymity as well as a secure communication between the whistleblower and the members of the Compliance department of Tecan in charge of investigating the issues reported. Tecan updated internal procedures and training modules in order to take into account opportunities and obligations related to the whistleblowing reports delivered over the EQS tool.
Tecan received 17 reports via the whistleblower platform in 2024, which corresponded to 15 cases. Of these, three cases progressed to in-depth investigation and corrective actions. The rest pertained to HR and behavioral topics or simple advice. To date, nine cases have been resolved whilst eight are still in progress. The higher number of reports received in 2024 in comparison to the six received in 2023 has been attributed to a global awareness-raising initiative within Tecan, increasing employees’ awareness of the hotline. The anonymity of reporters is protected, nonetheless based on the topics reported it is reasonable to conclude that no reports were made to the hotline by parties external to Tecan.

RISK MANAGEMENT PROCESS
To ensure sustainable corporate growth, it is crucial that any risks that could compromise this growth be recognized early on, assessed in terms of likelihood and consequences, and mitigated through an appropriate plan of measures. Tecan has a well-established global risk management process for this purpose with clearly defined roles and responsibilities at the Board of Directors, Management Board and other relevant leadership positions.
The process encompasses, among other factors, strategic risks, product risks, market and customer risks, occupational safety risks, risks relating to Tecan’s social and environmental impact and risks associated with the impacts of climate change. It also focuses on political and economic developments as well as the possible impacts certain events may have on external partners such as customers or suppliers. Tecan continuously adjusts its risk management system in line with changes to the environment and takes current events into account in its risk assessment. Business continuity planning is designed to ensure Tecan’s ability to withstand supply chain interruptions. The Board of Directors reviews annually whether the risk assessment of business activities is appropriate and whether it takes into account both internal and external changes. Where necessary, new measures to mitigate risk are implemented. Tecan’s risk management system is also regularly audited by a key insurer, who attests to the instrument’s high standard, enabling a premium reduction. Some of the company’s employees hold risk management certification, so the company does not have to depend exclusively on external experts.
ANTI-BRIBERY AND ANTI-CORRUPTION DUE DILIGENCE
Tecan carries out regular detailed screening of its distributors and has established a separate process with the TMS (Tecan Management System) directive Distributors and Intermediaries Anti-Bribery Due Diligence for this purpose. In particular, the TMS directive requires that all Tecan distribution partners and their owners, directors and employees refrain from bribing representatives of governments or state-owned or private enterprises, or from taking bribes. It does not matter whether bribery is prohibited, tolerated or allowed in the countries in which business is being done. Bribes are prohibited irrespective of whether a bribe is connected to a specific act or omission or is granted or received with a general view to the future execution of duties. Bribes do not only involve cash payments but also mean, for instance, lavish gifts, hospitality and entertainment. Distributors and intermediaries need to ensure that their representatives and their sales force are trained and adhere to Tecan’s standards of doing business. Tecan’s Compliance department closely monitors the compliance of the business run through dealers and distributors. In particular, activity is focused on ensuring that all third party intermediaries explicitly commit to our Code of Conduct, demonstrate a sufficient understanding of it and pass background checks without issues of concerns (legal disputes, criminal investigations, etc.). These steps are automated through the ethiXbase platform, which ensures a solid audit track of the checks performed. This platform allows a “real time” detection of unethical behaviors which may potentially have been reported regarding our dealers and distributors in the press or in the dedicated data banks.
Tecan annually assesses all operations for risk related to corruption (100%, 23 organizations – some smaller entities provided joint reports), and in 2024 identified no significant risk. Tecan only generates a smaller portion of its sales in countries that have an increased risk of corruption according to the criteria of the organization Transparency International.
DATA PRIVACY
Tecan is committed to handling all information (including personal, technical and commercial information) which employees, customers and other stakeholders entrust to it with due care, in compliance with applicable laws and solely for the purposes for which the information was provided or generated. When processing personal information, Tecan pays particular attention to the principles of transparency, lawfulness, proportionality and accountability. Tecan’s Data Protection Governance Structure includes a certified Group Data Protection Officer who directly reports to Tecan’s Management Board. Data protection is also supported by an online, easily accessible Data Subject Request Portal through which data subjects can invoke the rights they enjoy under applicable data protection laws.
TAX POLICY
Tecan’s strict adherence to the Company’s ethical code, respect for the environment, and full compliance to applicable laws and regulations in all the jurisdictions where Tecan operates applies also to the Company’s approach to taxation. This is set out in the Tax Principles, shared here and also available at tecan.com. The Tax Principles are owned by Tecan’s CFO, who is responsible for ensuring compliance with these principles. Risks associated with tax are included in Tecan’s risk management process, and concerns can be raised through the whistleblower hotline.
TECAN'S TAX PRINCIPLES 2024
Tecan is a leading global provider of laboratory instruments and solutions in biopharmaceuticals, forensics and clinical diagnostics. It is our mission to contribute to the quality of life of humankind by enabling our customers to make the world and our community a healthier and safer place. We live our core business values “Ambition”, “Highest standards” and “Trust”. Our behavior is governed by strict adherence to our ethical code, respect for our environment, and full compliance to applicable laws and regulations in all the jurisdictions where we operate. This is no different when it comes to taxation, which is an integral element of our overall corporate social responsibility. Our Tax Principles are in line with our core business values and are designed to support Tecan in delivering its strategic and sustainability ambitions.
PRINCIPLE 1 SUSTAINABILITY & GOVERNANCE
Tax is a core part of Corporate Responsibility and Governance and is overseen by the Board of Directors (the “Board”). The Group’s Tax Principles are in line with the goals of the OECD/G20 Base Erosion and Profit Shifting project and with its core principles, coherence, substance and transparency as well as consider economic and social impacts. The Board of Tecan yearly reviews the effectiveness of the Tax Principles and maintains a sound system of risk management and internal control. The Board is updated annually on tax risks and adherence to the Tax Principles. Our Tax Principles are mandatory and apply to all the entities and employees of the Group.
PRINCIPLE 2 COMPLIANCE
We comply with the tax legislation of the jurisdictions in which we operate, adhering to both, its letter and spirit, and pay the right amount of tax at the right time. All tax returns, claims, elections, disclosures, and payments shall be made accurately and on time. The Tecan Group Transfer Pricing Policy is defined and implemented based on the internationally accepted arm’s length principle, as described by the international tax conventions and by the OECD Guidelines, and as implemented in local rules and regulations. Transfer pricing methods follow a thorough analysis of the functions, risks and assets of the parties to the transaction. To ensure that the Group complies with local tax laws in the jurisdictions in which it operates and that solid and responsible tax planning is undertaken, we seek for adequate personnel, resources, up-to-date expertise, training and systems, and develop tax awareness across Tecan functions and businesses. Regular trainings are provided to Tecan employees with respect to the relevant tax policies. The Group Tax department under the responsibility of the CFO is managing the Group's tax risks. The Group Tax department ongoingly monitors and flags tax risks to the relevant party within the organizational structure. In addition, to mitigate tax compliance risks, Tecan has implemented standardized processes that regulate essential aspects of tax compliance. These processes identify the people and areas responsible for each phase of tax management and specify all activities to be carried out for the preparation of tax returns and self-assessments. Due to their nature, tax matters can be very complicated especially in multi-jurisdictional contexts. Whenever there exists significant uncertainty around a tax issue, including different interpretations of the applicable law, Tecan will seek advice from external advisors and/or from tax authorities in all circumstances where such clarification is possible. Tax risk control and tax-related reporting are supplemented by procedures and all tax accounting and compliance matters are subject to regular internal and external audits that assure the integrity and reliability of the accounting information used in tax processes.
PRINCIPLE 3 BUSINESS STRUCTURE DRIVEN BY COMMERCIAL CONSIDERATIONS
Our business structure is driven by commercial considerations, is aligned with business activity and has genuine substance. Our tax planning is based on reasonable, solid interpretations of applicable law and is aligned with the substance of the economic and commercial activity of our business. We do not use tax havens or opaque corporate structures to hide or reduce the transparency of our activities. Our Tax Principles extend to our relationships with employees, customers and suppliers. We do not engage in arrangements whose sole purpose is to create a tax benefit.
PRINCIPLE 4 CONSTRUCTIVE AND PROFESSIONAL RELATIONSHIPS WITH TAX AUTHORITIES
It is the Group's aim to maintain constructive and professional relationships with local tax authorities, based on mutual respect, transparency and trust. We are open and transparent with tax authorities, responding to relevant tax authority enquiries in a straightforward and timely manner to assist in the evaluation of tax liability. Our tax strategy is continuously aligned with instructions, regulations, and guidance of tax authorities. We will not bribe or otherwise induce tax officials, government officials or ministers with the aim of obtaining more beneficial outcomes with respect to tax matters.
PRINCIPLE 5 TRANSPARENCY
We strive for a regular information to our stakeholders, including investors, employees, civil society and the general public, about our approach to tax and taxes paid. This includes:
- We make our tax strategy public, including details of governance arrangements and our approach to dealing with tax authorities.
- An overview of our group structure and a list of all entities, with ownership information and a brief explanation of the type and geographic scope of activities.
- Annual information that explains our overall effective tax rate, together with information on our economic activity.
- Information on financially-material tax incentives where appropriate, including an outline of the incentive requirements and when it expires.
Disclosures are made in accordance with the relevant domestic regulations, as well as applicable reporting requirements and standards such as IFRS Accounting Standards. Tecan files yearly country-by-country Reports with the relevant tax authorities, which are exchanged with tax authorities in other jurisdictions based on international agreements.
PRODUCT QUALITY AND SAFETY
Tecan’s main business activities are the research, design and development of own products, the final assembly of these at our production sites, and the related sales and service activities. Tecan markets products directly to end-users and as an original equipment manufacturer (OEM). The products manufactured by Tecan are used in laboratories for life science research, applied markets, clinical diagnostics, and medical devices. The largest product group comprises laboratory automation platforms, benchtop instruments, as well as instrument components and sub-modules. In addition, Tecan also develops reagents to bring full solutions to customers in certain areas. Customers depend on Tecan to produce solutions that facilitate reliable, reproducible results. As a business-to-business rather than business-to-patient company, Tecan does not handle patient data or clinical samples directly. Nonetheless, we never lose sight of the potential human impact at the end of the chain of activities we are a part of. Tecan’s products are serving regulated applications and markets, yet it is important to Tecan to go beyond legal requirements and strive for excellence in product quality and safety. This has been one of Tecan’s core competences since the Company’s founding, more than 40 years ago. The Company’s values: ambition, trust and highest standards, are embodied by Tecan’s central Quality and Regulatory organization (QARA). Tecan’s approach to product development is characterized by a deep understanding of quality and regulatory requirements globally. QARA colleagues collaborate with customers from an early stage, supporting the product development process in a series of structured stages that span the product’s entire life cycle, up to the point where it is withdrawn from the market. Tecan’s commitment to quality is described at tecan.com , with the Quality and Regulatory Solutions brochure at this link setting out the expertise that enables Tecan to build regulatory requirements into a product order and ensure optimal product quality. Our work in this area is governed by the Tecan Group Quality Policy and the supporting documentation. These internal documents are stored in Tecan’s internal document management system, and accessible to all employees.
Tecan facilities are subject to a number of audits conducted by regulatory authorities, testing houses, monitoring and certification agencies, customers, and Tecan’s own specialist teams. These experts inspect whether Tecan’s facilities comply with country-specific regulations and the Company’s internal standards for product and occupational safety, as well as health and environmental protection. These inspections also cover measures that Tecan has to implement if it fails to meet any requirements. In 2024, Tecan successfully hosted several external authority and QMS certification audits, all concluding with no major findings. Notably, our production facility in Penang, Malaysia underwent a US FDA inspection, resulting with zero observations. This achievement underscores our unwavering commitment to upholding high standards of compliance.
Tecan participates in the Medical Device Single Audit Program (MDSAP), which sets out a catalog of requirements for manufacturers of medical devices, drawn up by several participating countries. MDSAP aims to ensure that standardized audits are performed, in addition to covering all country-specific regulatory requirements. Thus, manufacturers of medical devices can gain access to several markets by means of a single audit. All (100%) of the Tecan production sites eligible to participate in MDSAP maintained certification in 2024.
Regulatory requirements are continually evolving globally. To ensure these requirements are understood and implemented correctly, Tecan maintains a robust program of regulatory intelligence monitoring. Through this program Tecan identifies new and upcoming drafts of regulations, participates in industry forums and on regulatory committees, and is an early adopter of new regulatory requirements affecting Tecan product lines.
Another focal point in Tecan’s regulatory efforts is supporting customers in the Partnering Business by making key documentation available for authorization applications for new diagnostic products. Furthermore, Tecan builds strong, regulatory partnerships in order to guarantee successful marketing throughout entire product life cycles.
In addition, Tecan continues to be an early adopter of new or revised standards to ensure our own product lines and OEM partners remain compliant.
The Tecan parent company and all production sites are ISO 13485:2016 and ISO 9001:2015 certified, all sales subsidiaries operate under certified quality systems including ISO 13485:2016 and in many cases also ISO 9001:2016. Tecan QARA teams are continuing initiatives to address scalability of the Quality Management System based on scope of business at each Tecan entity and to harmonize processes for the digital age where appropriate. Tecan is well positioned and ready to implement FDA’s updated Quality Management Systems Regulation incorporating by reference ISO 13485:2016 as the accepted quality system standard for medical device companies.
The matrix certification approach for market units in Europe, China and Australia contributes to the sustainability efforts of Tecan by enabling audits to be conducted through a reduced sampling plan rather than on a site-by-site basis each year, reducing both travel needs and associated carbon emissions. This coordinated certification method accommodates Tecan’s current and future Group structure with an increasing number of subsidiaries, benefiting both customers and Tecan. In addition, Tecan’s implementation of an electronic Quality Management System (eQMS) strengthens sustainability by reducing paper usage, streamlining document management, and minimizing energy and waste associated with traditional paper-based systems. The eQMS also supports more efficient processes and real-time tracking, further lowering resource use and contributing to a reduced environmental impact across operations.
Tecan follows a controlled process for product information and labeling that is mandatory for meeting a number of regulations and is described in the internal documents Global Product Labeling SOP and Development of APL Material. These documents do not include information about sourcing of product components. The Global Product Labeling SOP does include:
- Internationally recognized hazard standards/symbols
- Required regulatory information based on product type and areas of commercialization
- Instructions for safe use of the product
- Proper disposal of the product.
Tecan also has a post-market surveillance process that monitors and responds to input from regulatory bodies and any customer complaints or inquiries received. This is set out in the internal documents Customer Support: Helpdesk/Expertline and Complaint Handling Process SOPs.
QARA teams throughout Tecan ensure that the Global Labeling and Advertising SOPs and the post-market surveillance process are being adhered to in all (100%) cases. Customer concerns or questions regarding product information and labeling can also be addressed through customer sales contacts, Tecan’s customer services, or Tecan’s whistleblower hotline regarding environmental protection, handling of hazardous materials and their disposal, or endangering the health and safety of other persons.
In 2024, no such complaints were made to the whistleblower hotline and there were no incidences of non-compliance with the Global Labeling SOP. The post-market surveillance process functioned as intended: Tecan detected and voluntarily issued corrections to two product labels in accordance with these post- market procedures. In 2024, no incidents of non-compliance with regulations concerning the health and safety impacts of products and services were identified.
Tecan’s QARA team is organized at Group level to ensure ongoing improvements based on changes in regulations worldwide and monitoring of product quality, and for addressing customer complaints. The Company performs a global management review every year in which relevant data from all Group companies are reviewed centrally. The process assesses whether quality management is still optimized and effective to the legal requirements and regulations for the products and services supplied by Tecan. Tecan undertakes this review with regard to the individual national markets as well as from a Group-level perspective, in this way, progress is evaluated.
CYBERSECURITY
IMPACT
By prioritizing cybersecurity and privacy of data, organizations can enhance trust, protect sensitive information, and maintain a secure operating environment. Cybersecurity is a material topic for Tecan, and includes implementing measures to ensure the confidentiality, integrity, and availability of data (e.g., customer and employee data), and protecting this data from unauthorized access, use, or disclosure. Some of Tecan’s customers use certain products for purposes that entail the processing of personal data and sensitive health information. Tecan’s services do not have the primary business purpose of processing such data on behalf of its customers. Nevertheless, Tecan may incidentally and unintentionally be exposed to such data. Tecan has processes and safeguards in place to address and mitigate the risks for the data subjects concerned in such instances.
Tecan embeds cybersecurity in its product development processes and business operations. Expert teams systematically manage information security and related risks throughout and coordinate, as needed, with Tecan’s Group Data Privacy Officer.
AWARENESS
A key aspect of Tecan’s cybersecurity strategy is raising awareness amongst all employees of the significance of a cybersecure environment. The foundation of a robust Tecan cybersecurity posture begins with educating our employees about potential cyber threats and the best practices to mitigate them. This is a critical element in protecting individuals and safeguarding digital assets of Tecan. We train employees with the aim of equipping them to recognize and respond appropriately to various forms of cyber threats, such as phishing, ransomware, and social engineering attacks.
We recognize that, even with the most effective preventive measures in place, cyber incidents can still happen. To address this, Tecan has a well-defined incident response plan in place. This plan outlines the steps to be taken in the event of a cyber incident, including how to contain the breach, assess and mitigate the damage, and notify affected parties.
Dedicated employees train to handle such emergencies by engaging in tabletop exercises; simulations of cyber attacks and incidents that provide hands-on practice and help them understand their roles and responsibilities during an actual cyber attack. A well-defined and practiced response plan will help us to minimize the impact of a cyber incident and expedite the recovery process.
POLICIES, DIRECTIVES AND ISO 27001 CERTIFICATION
In the realm of cybersecurity, the implementation and adherence to robust policies and standards are fundamental to an organization’s security infrastructure. These policies serve as the foundation for protecting against cyber threats and ensuring the integrity, confidentiality, and availability of data. A key component of these policies and standards is alignment with internationally recognized frameworks such as ISO 27001. ISO 27001 is a globally acknowledged standard for information security management systems (ISMS). It provides a systematic and well-structured framework that helps Tecan protect and manage its information through effective risk management. In 2024, Tecan achieved certification of compliance with ISO 27001, at a key site, demonstrating a commitment to maintaining a secure and efficient ISMS. Tecan’s global alignment with ISO 27001 guidelines not only enhances our cybersecurity posture but also instills confidence amongst stakeholders regarding the safeguarding of sensitive data and information systems.
DATA PRIVACY AND DATA PROTECTION
Tecan is committed to upholding the highest standards of privacy and data protection. In line with this commitment, Tecan embraces the principles of data protection laws such as the European Union’s General Data Protection Regulation (GDPR), which represents a significant milestone in data protection laws. The GDPR sets forth rigorous guidelines and practices for the handling of personal data, ensuring the privacy and security of individuals within the European Union and the European Economic Area.
Tecan has a Group Data Protection Officer (GDPO) who oversees our data protection strategy and compliance with GDPR requirements. The role of the GDPO underscores Tecan’s dedication to privacy and data protection. We continuously strive to maintain the highest level of data integrity and security, ensuring that our data handling practices not only comply with data protection laws but also align with the best practices in data protection.
DATA GOVERNANCE FOR AI AND AI GUIDELINE
Looking ahead, Tecan is actively embracing the potential of future technologies, with a particular focus on Artificial Intelligence (AI). As we venture into this domain, our approach is holistic, encompassing every aspect from governance to practical application. As we develop and deploy AI technologies, we keep ethical considerations and regulatory compliance front of mind. This includes establishing clear guidelines for AI usage, addressing issues such as data privacy, bias mitigation, and transparency in AI decision-making processes. At Tecan, we are excited about the future of AI and its transformative potential. Our approach is guided by a commitment to responsible innovation, ensuring that as we harness the power of AI, we do so with a focus on creating value for our customers and society at large.
IT AT TECAN
IT systems are always in focus of potential cybersecurity events. Tecan’s Global IT team operates a robust enterprise application landscape centered around an SAP core platform, which integrates sales, customer service, production, and financial processes into a unified system. Complementing this is the adoption of Microsoft M365 and cloud solutions, providing collaboration tools, secure communication, and additional flexibility to support modern business operations. Together, these platforms enable a comprehensive "business intelligence reporting suite" with integrated planning modules, such as those for human resources or budgeting. A continuous lifecycle for updates ensures that Tecan always has the latest software versions, thus limiting outages and helping avoid large-scale, expensive update processes with long test phases.
All main IT infrastructure services offered by the Group worldwide are outsourced and hosted to servers of an external service provider. The data is backed up redundantly, and the data centers are physically separated from one another and from the production sites. This enables Tecan to minimize the risk of critical data loss and increase data security. Global IT support is also available for Tecan sites in all regions, thereby reducing outages.
Tecan carries out regular cybersecurity audits, and related training is mandatory for all Tecan employees, with employees in key roles or demonstrating need receiving additional training. In 2024, 96.3% of all employees with access to the Learning Services Organization (LSO) platform and 83.5% of all contractors with access to the LSO platform at Tecan completed Cybersecurity training. Just over 40% of Tecan employees have access to a learning platform that is separate to the LSO and carry out their cybersecurity training there. Our training % data currently doesn’t include these employees, and we expect to have combined training data in future. The success rate of phishing simulations is tracked and forms the basis for follow-up where needed. This rate was satisfactory for Tecan in 2024 and is not disclosed as to do so is considered to be more likely to detract from rather than enhance Tecan’s cybersecurity efforts.
RELEVANCE TO TECAN’S PRODUCTS
Tecan continues to emphasize the importance of product security to ensure that customer use of Tecan products within a connected environment is not compromised and does not pose a security risk to end-user infrastructures. As the importance and benefits of global connectivity and open digital ecosystems become more widely appreciated, equally important is to develop secure digital offerings.
A Secure Software Development Lifecycle (SSDLC) process is essential so that security requirements are considered and introduced early on in product development and maintained throughout the product lifetime.
Tecan has identified IEC 81001-5-1: Health software and health IT systems safety, effectiveness and security Part 5-1: Security Activities in the product lifecycle as a state-of-the-art framework to ensure proper secure design of products. Tecan continues to work towards embedding 81001-5-1 requirements within the global Quality Management System. Of note within 81001-5-1, important techniques such as threat modeling, security risk assessment, security/penetration testing and vulnerability management will be further utilized in product development and lifecycle as standard practices. Respective measures and evidence will be part of R&D milestone documentation and templates.
Tecan’s design and development process is being enhanced to further align with 81001-5-1 requirements and best practices. Additionally, the security risk management process will continually evaluate security risks at a product level to determine if there are changes within the security risk profile of Tecan products. Processes utilized for secure design and maintenance are also subject to authority, certification body and/or internal audit review. To date there has been no reported exploit of a Tecan product which represents a serious incident. Tecan’s vulnerability management process will continue to monitor products for potential exploitability and is closely linked to remediation actions and improvements to further harden product’s security.
In addition to process enhancements to meet IEC 81001-5-1 requirements Tecan has conducted a portfolio evaluation and determined a product transition plan for existing products to become compliant with IEC 81001-5-1 as applicable. Product user information will continue to be enhanced to inform users how to setup Tecan products securely. Ongoing evaluation of new regional security regulations such as NIS2, Cyber Resilience Act will be incorporated into the respective processes accordingly.
INNOVATION
Tecan recognizes the importance of innovation to our long-term business success. In this context, "innovation" refers to product and service innovation, from improvements to disruptive or breakthrough innovations. This includes the R&D (research and development) activities undertaken to innovate products and services, as well as business model adaptations that might better satisfy customer needs and / or be aligned with sustainability challenges.
SOFTWARE
Innovation is an area in which Tecan stands out, particularly with regards to software development. Tecan has created a software architecture that allows us to cater to customer needs across a range of application areas, reflecting our unique end-to-end “from research to the clinic” product capabilities. Our software offering is modular, able to span a breadth of usage from industrial large-scale workflow hardware solutions to smaller benchtop solutions, closer to the direct patient environment. This modularity aspect of both hardware and software gives us the opportunity to use a common R&D and operational footprint in many different application areas, covering both Tecan’s Life Sciences Business and the Partnering Business world.
Tecan develops digital solutions that meet the needs of the regulated IVD and research markets. New products have been commercialized (with a SaaS business model) in the areas of service and management of entire instrument fleets. Our latest offering is a completely new software suite for laboratory orchestration. Tecan provides software solutions to increase personnel and instrument effectiveness by orchestrating the entire laboratory workflow, including hardware and configurations in these labs for Tecan and non-Tecan instruments. An open ecosystem has been created that enables Tecan to be at the forefront of application method development, for example for research and pharmaceutical laboratories as they strive for efficiencies. This innovative solution offers great potential for further expansion and new solutions to support our customers' digital transformation journey.
GRASSROOTS INNOVATION AND PRODUCT DEVELOPMENT
In 2024, Tecan’s grassroots innovation programs continued: the Time-boxed Innovations program, initiated in 2014, included several new approved ideas and resulting patent filings. In April 2024, Tecan held its second hackathon for employees, fusing the intensity of a brainstorming session, the dedication of a coding marathon and the spirit of innovation, bringing together the brightest coders from R&D. The primary goal of the hackathon was to foster collaboration among R&D teams and ignite their creativity, free from the constraints of daily routine. Eight teams with colleagues from five sites (Americas, Asia, Europe) were formed and worked together for three days, with the winning idea focused on a novel approach in the usability area. The event was a resounding success, generating great enthusiasm for future sessions.
Tecan’s approach to innovation is reflected in successful product launches, with a key example from 2024 being the Resolvex i300. The Resolvex i300 is a solid phase extraction module revolutionizing proteomic workflows by consolidating sample prep, cleanup, evaporation and resuspension tasks into one seamless operation. Complemented by our innovative AffinEx™ affinity purification columns and digital tools like Introspect™ and LabNavigator™, Tecan empowers the transition to a fully automated, error-reduced, and ROI-driven lab environment.
Our new Spark© Cyto 3DAI analysis tool also reflects the way Tecan enhances existing products with innovation and cutting-edge technologies. This new software plug-in enables 3D cell biology powered by AI, allowing the monitoring of key parameters for growing spheroids or organoids in real time.
During a Capital Markets Day in October 2024, Tecan previewed Veya, a multiomics liquid handling workstation that simplifies lab automation and boosts productivity. Officially launched at the Society for Laboratory Automation and Screening (SLAS) international conference in San Diego, USA, in January 2025, Veya provides effortless automation by overcoming key barriers in lab automation.
R&D PROCESSES
Tecan’s R&D development process was previously improved by integrating sustainability topics into existing stages such as the milestones review meetings, which is where project alignment with Tecan’s sustainability strategy is described. This alignment includes consideration of opportunities to design out waste, specify the use of materials with recycled content, and adopt lower impact packaging. In 2024, a checklist was added to this process and presented to R&D colleagues along with industry best practices in ecodesign including close collaboration with procurement colleagues and adopting a total cost of ownership approach when considering future products and materials.
As described in the Cybersecurity section of this report, R&D processes include strengthening cybersecurity controls, to ensure compliance with IEC 81001-5-1.
Protecting our intellectual property is of importance in ensuring that the development of new products and technologies gives Tecan a sustainable advantage in the market. Tecan registers patents on relevant developments for the most important markets in a timely manner and has several hundred patents in various patent families. In 2024, Tecan filed 18 patent applications on new inventions; one utility model patent, and one design patent. An overview of the various patents has been published on Tecan’s website.
Tecan has an internal guideline to support colleagues in their responsible use of AI tools. “Innovation” at Tecan is not governed by a stand-alone policy, the goals, targets and indicators used to track process in this area are embedded in the wider R&D processes. The effectiveness of Tecan’s approach to R&D is tracked internally, and lessons learned are incorporated into Tecan’s procedures. This information is not shared externally, for reasons of business confidentiality.
DIGITAL INNOVATION AND TRANSFORMATION OFFICE
In January 2024, Tecan established a Digital Innovation & Transformation Office, to expand our successful digital strategy in close collaboration with all relevant functions and stakeholders. Co-led by two newly created roles reporting directly to the CEO, VP Digital Innovation and VP Digital Transformation, this strengthening of leadership in digital competency demonstrates that Tecan’s ambition goes beyond keeping pace: we aim to lead the change in this fast-moving domain and ensure that potential is turned into impact in innovative growth products as well as substantial improvement of business processes.
Over the past year, Tecan has made substantial progress in digital innovation, reinforcing our leadership in establishing an open digital ecosystem for laboratories. We have introduced products that provide automated fleet overviews and efficiently streamline all assay steps, thereby enhancing productivity and quality for laboratory personnel. Our laboratory automation solutions enable the latest AI-driven drug discovery applications that require highly relevant multi-modal data in a GenAI-powered iterative feedback loop. These initiatives underscore our commitment to driving progress and delivering value in the evolving landscape of laboratory technology digitalization. Furthermore, through the development of ai.tecan.com, we have enhanced productivity by enabling the responsible use of modern AI tools on Tecan's infrastructure, thereby optimizing work environments and fostering innovation.
RESPONSIBLE SOURCING
Tecan’s main business activities are the research, design and development of our products, the final assembly of these at our production sites, and our related sales and service activities. Tecan manufactures products that are used in laboratories for life science research, in applied markets and in clinical diagnostics as well as in the medical area. The largest product group comprises laboratory automation platforms, benchtop instruments, as well as instrument components and sub-modules. Our products add value to society, and as described in the Social Impact section of this report, we also add value in our role as an employer. In 2024, Tecan continued to work to ensure that we have positive impacts, and avoid any negative impacts, through our supply chain. Tecan’s Human Rights and Responsible Business Practices policy reiterates our commitment to the principles of the United Nations Global Compact (UNGC), including the protection of internationally proclaimed human rights, the elimination of all forms of forced and compulsory labor, and the effective abolition of child labor. Potential negative impacts in the supply chain include breaches of the UNGC principles, either by Tecan suppliers or stakeholders our suppliers work with. This policy is available on tecan.com. In 2024, 99.1% of all employees with access to the Learning Services Organization (LSO) platform at Tecan and 85.4% of all contractors with access to the LSO platform at Tecan who had been assigned the Human Rights and Responsible Business Practices policy training had completed this, to ensure they are aware of the purpose, content and requirements of this policy. Employees without access to the LSO platform carry out trainings that have been reflected in the "training hours" data reported in the Social Impact section of this report.
Tecan products tend to be associated with specific Tecan production sites, and the purchasing for those sites is led by the site manager. In spending terms, between 60% and 80% of Tecan’s purchase volume is typically sourced in the same region as the production site. As well as facilitating the development of trusted business relationships, this proximity enables Tecan to better manage cost efficiency, inventory needs, just-in-time delivery, freight cost, and quality aspects.
Tecan’s Responsible Sourcing policy is published on tecan.com. The policy is supplemented by a detailed Standard Operating Procedure (SOP) found in Tecan’s internal document management system, the TMS (Tecan Management System). The steps of the Responsible Sourcing program are illustrated in the diagram here and described further below.
The aim of the Program is to ensure Tecan sources products and services from suppliers who adhere to internationally recognized ethical standards, and to avoid suppliers who engage in practices that are harmful to people or the planet.
The policy sets out how Tecan works to create a more responsible and sustainable supply chain that promotes social and environmental responsibility throughout the value chain. The process and instruments of the policy are as follows:
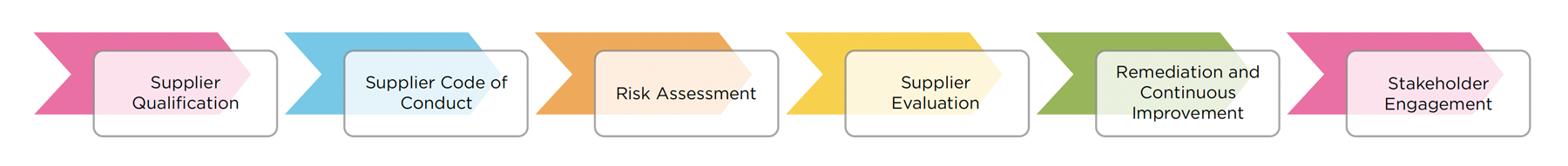
SUPPLIER QUALIFICATION
The environmental, social and governance practices of prospective suppliers are now assessed along with factors such as supplier’s production capabilities and quality management processes, in one comprehensive qualification process. This assessment includes evaluating the supplier’s management of environmental, social and governance risks in their own supply chain, thereby capturing all upstream economic operators. In 2024, the sustainability practices of 412 current suppliers were evaluated via a requalification process, using the same standards as the qualification process for new suppliers. In both processes an initial risk screening is carried out using a third party tool.
Suppliers are asked to share documents such as their own Supplier Code of Conduct, and to outline their procedures for managing environmental, social and governance risks associated with their supply chain and with their suppliers’ and subcontractors’ practices. Supplier qualification and requalification are not complete until the supplier has been approved by Tecan’s Responsible Sourcing Manager.
SUPPLIER CODE OF CONDUCT
Our Supplier Code of Conduct outlines the minimum standards we expect suppliers to meet in areas including human rights, labor practices, and environmental impact. Tecan seeks to only work with suppliers that meet or exceed the standards set out in our Supplier Code of Conduct. All new suppliers are requested to sign Tecan’s Supplier Code of Conduct as part of the supplier qualification process. Current suppliers are requested to sign the Supplier Code of Conduct as part of requalification.
RISK ASSESSMENT
A risk assessment screening of suppliers is carried out by performing a thorough due diligence to identify potential environmental, social and governance risks within the value chain, including risks relating to bribery and corruption, human rights, labor standards, child labor, environmental impact, and risks associated with conflict mineral import and processing. The assessment takes into consideration several factors including geographical location, industry sector, and supplier significance to Tecan. The assessment may include asking suppliers to provide additional corporate information and documentation demonstrating their environmental, social and governance practices.
SUPPLIER EVALUATION
Following the initial risk assessment, suppliers presenting a higher potential risk of inadequate environmental, social and governance practices are asked to provide additional information about their compliance with applicable laws and regulations, labor practices, environmental performance, and adherence to international standards and guidelines. Supporting evidence may also be requested, including ISO certifications and ratings such as that provided by EcoVadis. It is at this stage that on-site visits to suppliers would be most likely, followed by external audits if necessary. Tecan may conduct audits, assessments, or third-party verifications of our suppliers to evaluate their compliance with our Supplier Code of Conduct. Depending on the results of these steps, Tecan may require a supplier to improve their practices, or might decide not to work with the supplier.
REMEDIATION AND CONTINUOUS IMPROVEMENT
If any non-compliance or potential risks are identified through the risk assessment, supplier evaluation process or audits, including the risk of conflict minerals or child labor in the supply chain, Tecan may work with suppliers to develop and implement corrective action plans. We support their efforts to remedy any issues and promote continuous improvement in areas such as human rights, labor practices, and environmental sustainability by offering guidance and training. If a supplier does not improve their practices, Tecan is likely to terminate the supplier relationship. Tecan’s Responsible Sourcing program includes an escalation process to address cases in which a decision to terminate a supplier relationship could impact business continuity. This process includes stages culminating, if necessary, with a final review and decision by the appropriate Management Board Business Lead, Head of Operations, and Chief Technology Officer.
STAKEHOLDER ENGAGEMENT
Any stakeholder seeking to report issues relating to Tecan’s Responsible Sourcing policy or any breach of the standards set out in Tecan’s Code of Conduct can do so via Tecan’s whistleblower hotline, accessible at tecan.com. This dedicated hotline ensures the highest standards of confidentiality and anonymity as well as a secure communication between the whistleblower and appointed specialized members of Tecan in charge of investigating and processing the issues reported.
TRANSPARENCY AND REPORTING
Tecan is committed to transparency and reporting on our Responsible Sourcing activities. We communicate our progress and challenges in managing supply chain risks to relevant stakeholders, including employees, customers, and investors, in our Annual Report.
IMPACT OF TECAN’S RESPONSIBLE SOURCING PROGRAM
The Data section of this report includes the results of Tecan’s Responsible Sourcing program. The program includes assessing the risk of conflict minerals or child labor issues in Tecan’s supply chain in accordance with the Swiss supply chain legislation (DDTrO). In 2024, it was reconfirmed that Tecan does not import into Switzerland or treat in Switzerland materials that fall within the scope of this legislation. Child labor risk is included in the steps of the program described above.
